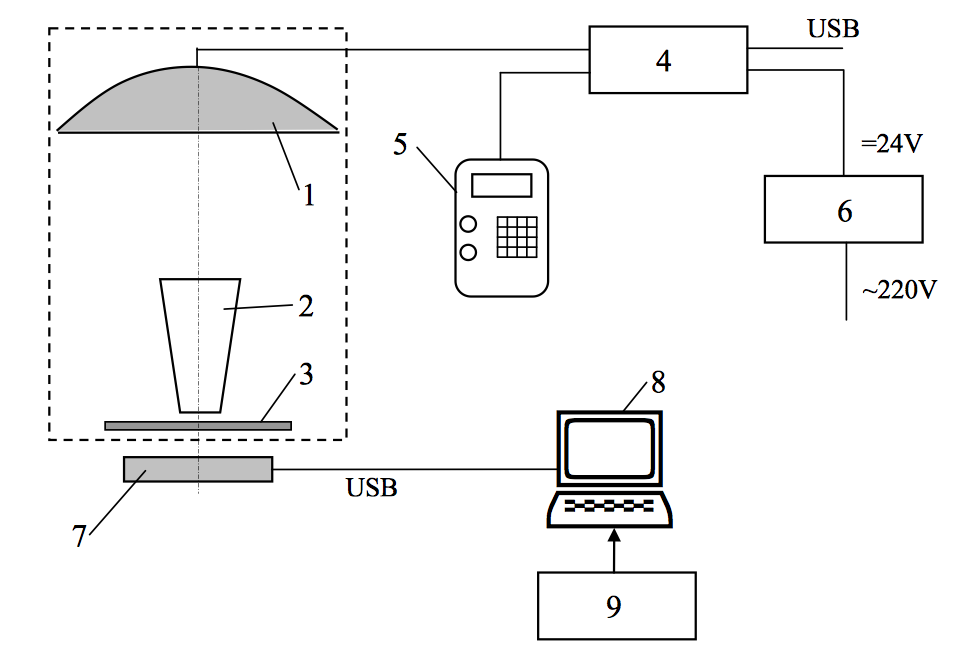
The trial model of the system for oncological disease treatment with photodynamic therapy method.
The treatment of the oncological diseases with photodynamic therapy method (PDT) is based on the cancerous cells selective destruction owing to the residues of photochemical reactions taken place into the malignant tumors those have accumulated photosensitizer selectively when dosed exposing of the light with specific wavelength. The malignant tumors destruction takes place under reach the appointed photodynamic dose (50-100 J/cm2) that is determined both irradiation power density level at the spectral region of the photosensitizer activation and treatment procedure period directly.
The experimental model of the clinical apparatus for the treatment of oncological diseases with photodynamic therapy method (CAPTOD-PDT) uses the continuous mode for the tissue irradiation. This mode can be realized with bulk light emitted diodes using (1000-1500 pieces). The domestic photosensitizer Hyperflav (SIC “Borscchahivskiy chemical-pharmaceutical plant” CJSC is its developer and manufacturer) has an absorption spectral maximum at the 595±3 nm wavelength. To exposure operating surface on the tissue matrix irradiating source on the base of superluminiscent LEDs of 510MY8C series (Hebei Ltd., China R.P.) have been applied. Selected LEDs have an irradiating maximum at the λmax = 588 nm wavelength, high light intensity values (6000-10000 mccd), the narrow spectral half-width (Δλ0.5 = 35-40 nm) and narrow radiation pattern (2θ = 12˚-15˚).
The main task is ensuring the irradiation power density on the operation zone required for PDT treatment and has been resolved with optical distal instrument use. That has been made in a hollow mirror frusta conical shape with cone big basis turned to the matrix irradiating source. Then the operation zone is coinciding with cone small basis. The matrix irradiating source has been fabricated in the spherical segment shape with the LEDs ranged on it. The electric network of LEDs’ operating has been planned their conjunction as the clusters (24 clusters with 48 LEDs each and 8 edge ones with 24 LEDs each). Also CAPTOD-PDT includes 24V output power supply has up to the clinical use quality, control console, PC connected photodynamic dose pickup unit.
The CAPTOD-PDT technical regulations draft has been designed for purpose to its subsequent state registration as the medical device. That enables to use CAPTOD-PDT in the clinical practice in Ukraine.