Capacity SiO2/Al2O3-cathode of lithium power sources depending on the energy redistribution of the electrons caused by the conditions of synthesis of nanocomposites
The charge capacity of cathodes of lithium power sources (LIPS) depends on the degree of implementation of the ions in the structural channels, defects and other features of the developed surface of nanosized materials. Interaction ions of lithium with the ions which are on the surface nanoparticles directly dependent on the energy distribution of the valence electrons, particularly a negatively-charged ions. It is important to identify change of these characteristics as a result of methods of synthesis of nano-sized composites. The results of the research of the electronic structure of component of nanocomposites and their mixtures revealed a significant dependence of the charge state of the ions on the composition and differences in their chemical potentials. Thus, the beat set, instability of the charge capacity during cycling LIPS with cathodes based on mixtures obtained from the conventional stirring. The analysis of study the electronic structure of these compounds have not found changes in the energy distribution of the valence electrons in relation to the original components, it not detected the presence of electrons in the disconnected states anions which easily captures lithium ions. Ions are therefore not converted into atoms and do not form the oxide film.
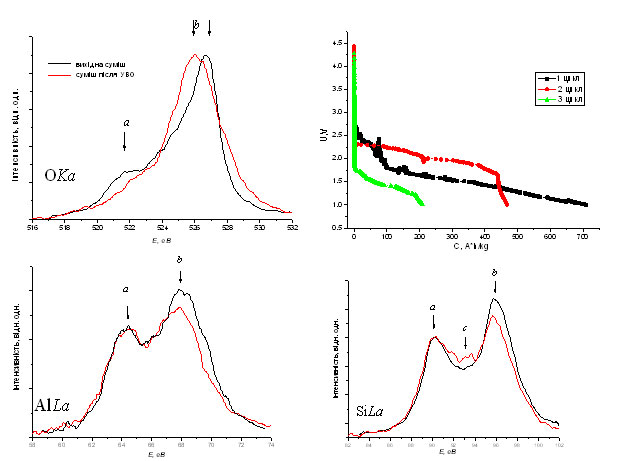
The difference between the chemical potentials of components contributes interatomic interaction between surface atoms of the neighboring nanoparticles under certain conditions, their treatment, which would allow a significant contact between nanoparticles and the overlap of wave functions of the valence electrons on their surface. Studies of transmission electron microscopic images with high resolution of mixtures after the shock vibration treatment revealed association of nanoparticles. Investigation of the energy distribution of the valence electrons of oxygen, silicon and aluminum shock-treated composite established emergence of Op-bonds on the interface and increasing charge of oxygen ions due to transfer electrons from the cations to further split Or-states.
Increased charge of oxygen led to increased charging capacity cathode LIPS on the basis of the composites. Increasing the charge due to the settlement only binding states led to the absence of recombination of lithium ions in interaction with oxygen anions. As a result, the charge capacity of the LDS increased by 200 A*h /kg each of the following cycles, because Li+ does not form oxide films on the surfaces of nanoparticles, unlike recombined atomic lithium.
Research nanocomposites obtained by synthesis pyrogenic method showed absence of patterns growth of charge oxygen depending on composition of composites, due to lack of control of the parameters synthesis.
Discovered mechanism of intercalation processes and their dependence on the electronic structure allows purposefully choose nanoscale materials and methods of producing composite for manufacturing cathodes LIPS with stable and high electrochemical properties.
| Attachment | Size |
|---|---|
| 730.48 KB |