Influence of the electronic structure of SiO2/TiO2/Fe2O3/Al2O3 nanocomposite cathodes obtained by shock-vibration treatment on the charge capacities of lithium current sources
For the intensive development of scientific and technological progress, it is necessary to use new materials with unique desired properties, such as high sorption ability, superconducting properties, high ability to accumulate electric charge, etc. Despite the intensive development of electrical engineering and an increase in the number of portable electronics, it is necessary to use portable sources energy with high rates of charge capacity and cyclability. Currently, one of the most promising is lithium current sources (LCS). Therefore, we can say with confidence that the search for new electrode materials that would provide the above features is undoubtedly an urgent task. It is known that the properties of materials, including the electrochemical properties of LCS, depend on the electronic structure of materials and the type of interatomic interaction of atoms in crystalline, amorphous, or molecular structures. But another structure is formed in the process of using certain methods of synthesis or processing. Therefore, to simplify the above task, it is very important to understand how the distribution of valence electrons, the morphology and structure of electrode materials change during their preparation. And the another one task the looking for the answer for the question - what is the relationship between these parameters and the electrochemical properties of LCS. Therefore, in this work, the influence of shock-vibration processing of mixtures of nanopowders of Al2O3, SiO2, TiO2 and Fe2O3 oxides on their electronic structure, structural and morphological features, and the charge of the LCS capacitance with cathodes based on them was established and the relationship between these characteristics was established.
The difference in the chemical potentials of the components contributes to the appearance of an interatomic interaction between the surface atoms of neighboring nanoparticles at high local pressures and temperatures as a result of treatment. This leads to an increase in the charge state of oxygen. If the charge state grows due to the population of highly energetic unbound states, this leads to an increase in the ability of lithium ions in recombination with surface oxygen atoms and the formation of oxide groups. These oxide groups on the surface of the cathode LCS material prevent the incorporation of lithium ions into structural channels and voids, which leads to the absence of cycling of such LCS. At the same time, the population of electrons in the low-energy region at the Opπ-binding level leads to a decrease in the recombination ability and, accordingly, such a material will have cyclic properties.
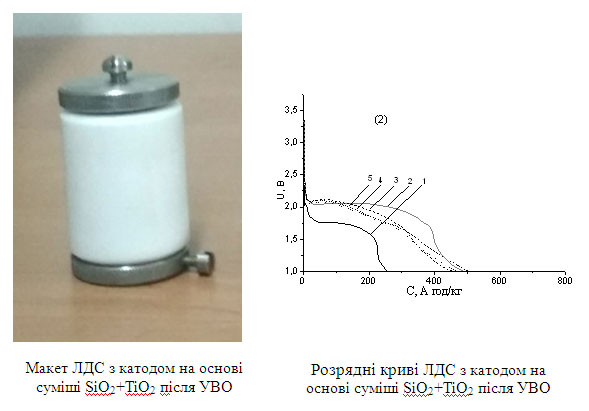
The discovered mechanism of inter and deintercalation processes and their dependence on the electronic structure allows one to selectively select nanoscale materials and methods for producing composites for the fabrication of LCS cathodes with stable and high electrochemical properties. From the conducted studies, it was found that the most promising nanocoposites for use as the basis for the cathode material are 0,2TiO2+0,8SiO2 and 0,2Al2O3+0,8SiO2 with sufficiently high specific capacities and capacities.
| Attachment | Size |
|---|---|
| 421.55 KB |